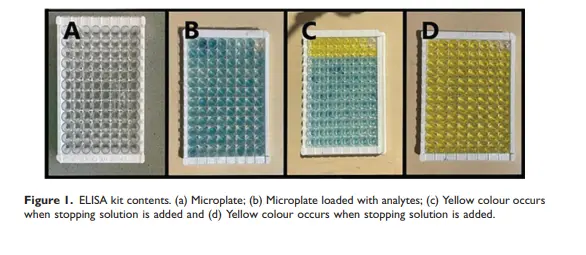
How to Run a Successful ELISA: From Protocol to Reliable Results
ELISA (Enzyme-Linked Immunosorbent Assay) is a powerful technique for detecting and quantifying target molecules such as proteins, hormones, or antibodies. Ensuring accuracy requires attention to detail at every step.
✅ Key Steps for Success
1️⃣ Plate Coating
– Use high-binding plates for consistent capture.
– Coat with antigen or antibody at the recommended concentration, typically overnight at 4°C or 1-2 h at 37°C.
2️⃣ Blocking
– Apply blocking buffer (e.g., BSA, casein) to prevent non-specific binding.
– Incubate for 1 h at room temperature.
3️⃣ Sample and Standard Application
– Dilute samples properly to fall within the linear detection range.
– Run standards in duplicate or triplicate for accuracy.
4️⃣ Detection Antibody
– Use validated primary and secondary antibodies at optimal dilutions.
– Incubate according to the protocol, usually 1 h at 37°C.
5️⃣ Substrate Addition
– Add TMB or other chromogenic substrate.
– Monitor color development carefully; stop reaction with acid at the right moment.
6️⃣ Read and Analyze
– Read absorbance at the correct wavelength (commonly 450 nm).
– Plot standard curve and calculate concentrations using appropriate software.
Learn more .
⚠ Common Pitfalls
– Uneven coating → use calibrated pipettes, avoid drying out wells
– High background → optimize blocking and washing steps
– Low signal → check antibody quality and dilutions
Figure 2: Schematic drawings of: (a) Direct ELISA; (b) Indirect ELISA; (c) Competitive ELISA; (d) Sandwich ELISA; (e) Direct cellular ELISA and (f) Indirect cellular ELISA.
PCR and qPCR: Mastering Amplification and Real-Time Detection
PCR amplifies DNA fragments; qPCR allows quantification in real time using fluorescence. Both demand precision for reproducible results.
✅ PCR Best Practices
1️⃣ Primer Design
– Ensure specificity (use BLAST search).
– Optimal Tm: 55–65°C; avoid secondary structures.
2️⃣ Reaction Mix Preparation
– Prepare master mixes to minimize pipetting error.
– Use nuclease-free water and certified reagents.
3️⃣ Cycling Conditions
– Optimize annealing temperature.
– Avoid excessive cycle numbers to reduce nonspecific amplification.
✅ qPCR Considerations
– Choose the right chemistry (SYBR Green or probe-based like TaqMan™).
– Run no-template controls to detect contamination.
– Verify amplification efficiency (90-110% is ideal).
⚠ Troubleshooting Tips
– Primer-dimer formation? Redesign primers or increase annealing temperature.
– Variability between replicates? Improve pipetting accuracy, mix thoroughly.
– No amplification? Check template quality, enzyme activity.
📘 Western Blot Troubleshooting: From Gel to Image
Western blot is essential for protein detection and quantification. To obtain clear, interpretable results, avoid common errors.
✅ Critical Steps
1️⃣ Sample Preparation
– Use fresh lysates; quantify protein concentration.
– Load equal amounts (e.g., 20–50 µg per lane).
2️⃣ Gel Electrophoresis
– Choose appropriate gel % for target protein size.
– Run under consistent voltage to avoid band distortion.
3️⃣ Transfer to Membrane
– Confirm transfer efficiency by staining (e.g., Ponceau S).
– PVDF or nitrocellulose? Choose based on target protein and detection method.
4️⃣ Blocking and Antibody Incubation
– Use suitable blocker (milk, BSA) for your antibody type.
– Optimize primary and secondary antibody dilutions.
5️⃣ Detection
– Use fresh substrate; minimize exposure to prevent oversaturation.
– Include molecular weight markers for reference.
⚠ Common Issues
– High background? Increase washing, adjust blocking conditions.
– Weak bands? Verify antibody quality, increase protein load.
– Multiple bands? Check specificity, reduce antibody concentration.
📘 Best Practices for Safe Handling of Pharmaceutical Chemicals
Working with pharmaceutical-grade chemicals demands strict safety measures to protect personnel and ensure product integrity.
✅ General Principles
– Always consult the Material Safety Data Sheet (MSDS) before use.
– Wear appropriate Personal Protective Equipment (PPE): gloves, lab coat, eye protection.
✅ Handling and Storage
– Store chemicals according to hazard class (e.g., acids separate from bases).
– Label all containers clearly, including date of receipt and opening.
– Work in a fume hood when handling volatile or toxic substances.
✅ Spill and Waste Management
– Have spill kits readily available and train staff on their use.
– Segregate hazardous waste, and dispose of via certified services.
✅ Documentation
– Maintain usage logs for controlled substances.
– Keep updated inventory for audits and traceability.