ammonium chloride as acidifiers
Ammonium chloride (NH₄Cl) as an acidifier is commonly used to acidify solutions or environments, especially in medical, agricultural, and industrial applications.
🔬 How Ammonium Chloride Works as an Acidifier
- Ammonium chloride dissociates in water:
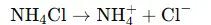
- The ammonium ion (NH₄⁺) is a weak acid and can release H⁺ ions through this equilibrium:
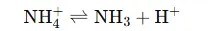
- This release of H⁺ lowers the pH, thus acidifying the solution.
⚙️ Common Uses of Ammonium Chloride as an Acidifier
-
Medical Use
- Treat metabolic alkalosis by acidifying blood plasma.
- Used in some cough medicines for its expectorant and acidifying properties.
-
Agriculture
- Added to animal feed to acidify urine, reducing the risk of urinary stones in livestock.
- Helps maintain soil pH in some conditions.
-
Industrial Applications
- In metal finishing and plating baths to control pH.
- In electrolytes for batteries.
🔎 Advantages of Using Ammonium Chloride
- Water-soluble and easy to handle
- Mild acidifying effect compared to strong acids
- Provides ammonium ions beneficial in some biological contexts
⚠️ Notes and Safety
- Overuse can lead to acidosis or irritation.
- Should be used carefully following recommended dosages.
- Handle with gloves and avoid inhalation of dust.